INNOVATIVE TECHNOLOGIES FOR MEDICAL TECHNOLOGY
Chronic diseases are rapidly increasing due to continuous demographic development and changing lifestyles of our society. Medical technology needs innovative technologies in order to diagnose and identify chronic diseases. At the AIT, Highly qualified, interdisciplinary teams are facing these challenges.
We are focusing on chronical diseases in order to develop a more effective diagnosis and treatment program for stationary areas. In order to improve the therapeutic consistency of medication, we are working in close collaboration with pharmaceutical businesses, their suppliers and CRO's, focusing on approval and observational studies as well as the daily application through the patient.
// Algorithms and bio-mathematical models
We develop innovative algorithms and bio-mathematical models to improve differential diagnostics and to predict cardiovascular diseases. Our algorithms are based on our ARCSolver® technology, licensed to the medical technology industry, focusing on diagnostic functions for professional clinical areas.
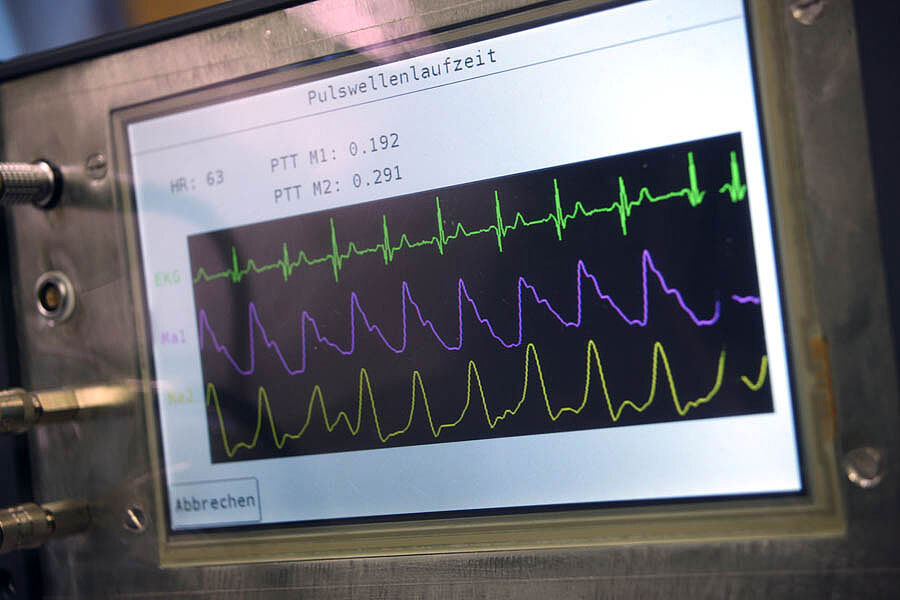
// Pulse wave analysis and vascular age measurement
We highly value research of new medical indications (focusing on cardiology, hypertensiology and nephrology) by combining the pulse wave analysis with electrophysiology. Considering this progress, we are including new initiatives for space medicine.
// Therapy support and medical adherence
Based on professional clinical areas, we adapt our existing technologies to patients and consumers. We are specifically focusing on therapy support and adherence during medication, for example when managing diabetes and high blood pressure.
// Research Services
- Recording and analysis of bio-signals (PWA, EKG, …)
- Statistical study support
- Support in study concept development
- Requirement Engineering inkl. user consultation
- Design Studies for medical devices (hard- and software), including 3D print and Usability.
- Development of elektronic medical devices according to ISO 60601-1 corresponding to MDR and IVDR
- Development of medical Software (Apps) corresponding to MDR and IVDR
- Measurement and validation during product development