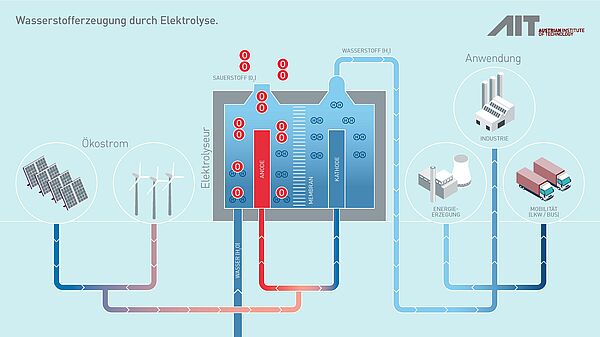
Electrolyzer: key component in the renewable energy system
An electrolyser breaks down water into its basic components, hydrogen (H₂) and oxygen (O₂), through the process of electrolysis.
Electrolysis
Water electrolysis, the electrochemical splitting of water into hydrogen and oxygen using electricity, currently only accounts for around 0.1% of global hydrogen production. However, with announced and planned projects, global electrolyzer capacity could reach between 175 and 420 GW by 2030.
Electrolyzer: Research and development services
The AIT Austrian Institute of Technology supports the further development of electrolysis technologies through highly specialized research and development services. The focal points include:
1. Electrolysis
The AIT works on improving the efficiency and cost-effectiveness of electrolysers. The focus is on innovative solutions for water electrolysis that make it possible to produce green hydrogen on a large scale and using renewable energy sources.
2. Development and validation of materials, components and systems
AIT offers comprehensive expertise in the development of new materials for electrolyzers as well as in the validation of complete systems. This includes the optimization of components to improve the performance and lifetime of electrolysers.
3. Development of electrode materials
Research at the AIT focuses on the development of high-performance electrode materials that can make the operation of electrolysers more efficient and sustainable. The aim is to optimize electrochemical processes and reduce material costs at the same time.
4. Development of new technology concepts
In addition to the further development of existing technologies, the AIT is working on researching completely new concepts for electrolysis plants. These aim to increase efficiency, minimize resource consumption and facilitate integration into renewable energy systems.
Types of electrolyzers
Electrolyzers play a crucial role in the production of green hydrogen, particularly when powered by renewable energy sources such as wind or solar energy. There are several types of electrolyzers, including:
- Alkaline Electrolyzer – Uses an alkaline solution as the electrolyte.
- Proton Exchange Membrane (PEM) Electrolyzer – Utilizes a solid proton exchange membrane to transfer protons (H⁺) from the anode to the cathode.
- Solid Oxide Electrolyzer (SOEC) – Operates at high temperatures and uses a solid ceramic material as the electrolyte.
These technologies are vital for advancing the adoption of hydrogen as a clean energy carrier.
Market Development for Electrolyzers
At the end of 2022, 60% of the installed electrolyzer capacity consisted of alkaline electrolyzers, followed by Proton Exchange Membrane (PEM) electrolyzers, which accounted for approximately 30%. This distribution is expected to shift in the coming years, as PEM electrolyzers are projected to gain market share over alkaline electrolyzers. This forecast is based on numerous project announcements, though many future projects have not yet decided or disclosed which electrolyzer technology they will use. Solid Oxide Electrolyzers (SOECs) currently play a minor role, with less than 1% of the installed capacity. In terms of geographical distribution, as of the end of 2022, one-third of the world's installed capacity was located in China, another third in Europe, while the USA and Canada together accounted for about 10%.
Alkaline Electrolyzer (AEL)
The alkaline electrolysis (AEL) is one of the most established methods for hydrogen production and plays a crucial role in the energy transition. As a key technology for producing green hydrogen, it offers numerous advantages, particularly due to its proven reliability and durability in large-scale industrial applications.
What is Alkaline Electrolysis?
Alkaline electrolysis is a process that splits water into hydrogen and oxygen. Electric current is passed through an electrolyte composed of water and a basic chemical such as potassium hydroxide (KOH) or sodium hydroxide (NaOH). AEL has proven to be robust and efficient in industrial applications, offering a relatively simple design, long lifespan, and scalability. These characteristics make it particularly suitable for industrial hydrogen production.
How Does Alkaline Electrolysis Work?
The electrolysis process involves two main reactions: oxidation at the anode and reduction at the cathode.
- Anodic Reaction (Water Oxidation): At the positively charged anode, hydroxide ions (OH⁻) from the electrolyte are oxidized, producing oxygen gas, water, and electrons. The reaction is:
4OH⁻ → O₂ + 2H₂O + 4e⁻ - Cathodic Reaction (Water Reduction): At the negatively charged cathode, water molecules are reduced by incoming electrons, generating hydrogen gas and regenerating hydroxide ions that return to the electrolyte:
4H₂O + 4e⁻ → 2H₂ + 4OH⁻
Advantages of Alkaline Electrolysis
Alkaline electrolysis offers several benefits, making it one of the most widely used technologies for hydrogen production:
- High Reliability and Durability: AEL technology is particularly reliable and durable due to its robust design and proven materials. AEL electrolyzers can operate continuously over extended periods without significant performance degradation.
- Cost Efficiency: AEL electrolyzers are relatively inexpensive compared to other electrolyzer technologies, such as PEM electrolyzers. Large-scale production facilities benefit from the economic efficiency of AEL.
- Industrial Applications on a Large Scale: AEL systems can produce large amounts of hydrogen, making them attractive for industrial applications such as steel production, the chemical industry, and other energy-intensive sectors.
- Proven Technology: AEL is a well-established technology with a long history of successful use at an industrial scale, making it a reliable choice for hydrogen production.
Challenges of Alkaline Electrolysis
Despite its numerous advantages, alkaline electrolysis faces certain challenges that require further development and optimization:
- Lower Efficiency Compared to PEM: AEL has slightly lower energy efficiency than Proton Exchange Membrane (PEM) electrolysis due to higher electrical resistance and slower electrochemical reactions in the alkaline environment.
- Higher System Complexity: AEL requires additional components for electrolyte management due to the use of liquid electrolytes, increasing system complexity and maintenance requirements.
- Limited Flexibility with Renewable Energy: AEL responds more slowly to fluctuating energy inputs, such as those from renewable energy sources like solar and wind. This limits its integration into hybrid energy systems that require rapid adaptation to variable energy supplies.
Research and Development: Optimizing AEL Technology
Recent research has focused on improving the efficiency and performance of alkaline electrolysis. Key research areas at AIT include innovative materials and new design approaches:
- Enhanced Electrode Materials: New electrode materials can increase reaction rates at the electrodes and improve overall AEL performance. Nanostructured materials and advanced catalysts offer promising approaches to enhance AEL efficiency.
- Membrane Development: Researchers are working on optimizing membranes in AEL systems to improve ion conductivity and reduce electrical resistance, which could boost system performance.
- System Integration: Another research focus is integrating AEL into hybrid energy systems powered by renewable energy. Developing smart control systems and adapting AEL to variable energy inputs could further enhance hydrogen production efficiency.
Applications of Alkaline Electrolysis
The flexibility and robustness of alkaline electrolysis make it suitable for a wide range of applications:
- Large-Scale Industrial Hydrogen Production: Due to its high hydrogen production capacity, AEL is widely used in steel production, the chemical industry, and other energy-intensive sectors.
- Green Hydrogen: In combination with renewable energy, AEL can produce green hydrogen for use in sectors such as mobility, energy storage, and industrial processes.
- Energy Storage: Hydrogen produced by AEL can serve as an energy storage medium in hybrid energy systems, storing excess energy from renewable sources for later use.
Future Outlook for Alkaline Electrolysis
Alkaline electrolysis has established itself as one of the most important technologies for hydrogen production, particularly for large-scale industrial applications. With ongoing research and development, AEL efficiency is expected to improve, and costs are anticipated to decrease.
The combination of AEL with renewable energy presents significant potential for large-scale green hydrogen production, contributing substantially to the decarbonization of industrial and energy sectors. Advances in materials science and system integration will be crucial for the further adoption of AEL technology in the coming years.
PEM Electrolyzer
What is PEM Electrolysis?
PEM (Proton Exchange Membrane) electrolysis is a hydrogen production process that splits water (H₂O) into hydrogen (H₂) and oxygen (O₂) using electrical energy. A proton exchange membrane serves as the electrolyte in this process. The membrane is permeable to protons but impermeable to electrons, enabling efficient separation of hydrogen and oxygen.
How Does PEM Electrolysis Work?
PEM electrolysis relies on an electrochemical process, divided into two main reactions:
- Anodic Reaction (Water Oxidation): At the positively charged anode, water is oxidized and split into oxygen, protons, and electrons. The reaction equation is:
2H₂O → O₂ + 4H⁺ + 4e⁻ - Cathodic Reaction (Water Reduction): At the negatively charged cathode, protons (H⁺) that have traveled through the PEM membrane are reduced with electrons from the external circuit to form hydrogen:
4H⁺ + 4e⁻ → 2H₂
The hydrogen generated during this process is then collected and can be used in various applications, including mobility, industry, and energy storage.
Advantages of PEM Electrolysis
Compared to other electrolyzer technologies, such as alkaline electrolysis, PEM electrolysis offers several advantages:
- High Efficiency: The proton exchange membrane enables high efficiency in hydrogen production.
- Fast Response Times: PEM technology responds quickly to fluctuations in power supply, making it ideal for use with renewable energy sources like wind and solar.
- Compact Design: PEM electrolyzers are compact and require less space than other technologies, facilitating integration into existing systems.
- High Hydrogen Purity: The hydrogen produced is of very high purity, which is crucial for certain industrial applications.
Challenges of PEM Electrolysis
PEM electrolysis faces several challenges, particularly in scaling up to meet the demand for green hydrogen:
- Catalysts: PEM electrolyzers use catalysts typically made from platinum group metals (PGMs) such as platinum and iridium. These materials are expensive and rare, driving up investment costs and limiting large-scale adoption.
- Electrolyzer Stack: The stack, consisting of multiple cells, requires costly components capable of withstanding acidic environments and high currents.
- Catalyst and Membrane Degradation: PEM electrolyzers operate in highly corrosive acidic environments, which can degrade catalysts over time, particularly at the anode. The proton exchange membrane is also susceptible to degradation under high voltage and variable load conditions, affecting system efficiency and lifespan.
- Water Purity Requirements: PEM electrolyzers require highly purified water, as contaminants like ions or particles can damage the membrane or poison the catalysts, reducing efficiency and lifespan. Additional water purification steps add to operational complexity and costs.
Research and Development: Advancing PEM Electrolysis
Research and development at AIT focuses on overcoming technical and economic challenges in PEM electrolysis to improve efficiency, durability, and scalability. Key R&D areas include:
- Reducing PGM Usage in Catalysts: Developing alternative catalysts that are less expensive and more abundant, such as PGM-free catalysts or those with reduced PGM content, while maintaining or enhancing efficiency and stability.
- Improved Membranes: Creating new membrane materials or composites that offer high proton conductivity and are resistant to degradation.
- Cost-Effective Materials: Reducing costs for other components, such as bipolar plates and end plates, to make PEM electrolysis more economical.
- System Design and Integration: Adapting PEM electrolyzers for dynamic load operations with renewable energy sources and integrating them into storage and fuel cell technologies.
Applications of PEM Electrolysis
The flexibility and efficiency of PEM electrolysis make it ideal for a wide range of applications:
- Green Hydrogen: When combined with renewable energy, PEM electrolysis can produce green hydrogen, an emissions-free energy carrier.
- Hydrogen Storage: Energy generated through PEM electrolysis can be stored as hydrogen and later reconverted into electricity, helping to balance fluctuations in renewable energy supply.
- Industrial Applications: Hydrogen is essential for numerous industrial processes, such as those in the chemical industry and steel production. PEM electrolysis provides a sustainable method to meet this demand.
High-temperature electrolyzer: efficient hydrogen production through thermochemical processes
What is High-Temperature Electrolysis?
High-temperature electrolysis (HTEL) is a promising technology for hydrogen production. Unlike other electrolysis methods, HTEL uses elevated temperatures to reduce the energy required for water splitting and enhance process efficiency. Particularly when combined with renewable energy sources or industrial waste heat, HTEL has the potential to make hydrogen production more sustainable and cost-effective.
High-temperature electrolysis is a process where water (H₂O) is split into hydrogen (H₂) and oxygen (O₂) at temperatures between 700°C and 1000°C. The input of thermal energy reduces the energy demand for the electrochemical process, enabling higher efficiency compared to other electrolysis methods such as alkaline or PEM electrolysis.
How Does High-Temperature Electrolysis Work?
This process involves two key reactions:
- Cathodic Reaction (Water Reduction): At the cathode (negatively charged electrode), water vapor is reduced to produce hydrogen gas and oxygen ions. The reaction is:
H₂O(g) + 2e⁻ → H₂ + O²⁻ - Anodic Reaction (Water Oxidation): At the anode (positively charged electrode), oxygen ions migrate through the electrolyte, release electrons, and combine to form oxygen gas:
2O²⁻ → O₂ + 4e⁻
Advantages of High-Temperature Electrolysis
Compared to other electrolysis methods, HTEL offers several advantages, making it particularly attractive for industrial applications and large-scale hydrogen production:
- Higher Efficiency: By utilizing thermal energy, HTEL achieves significantly higher efficiency than other methods. This allows for more effective use of energy sources, especially when using waste heat from industrial processes.
- Reduced Electricity Consumption: Since a substantial portion of the energy for water splitting is provided as heat, the electrical energy consumption is lower than in conventional methods, reducing operational costs.
- Potential for Industrial Integration: HTEL can be effectively integrated into industrial processes that generate large amounts of waste heat, such as steel mills, chemical plants, or power plants. Using this waste heat for hydrogen production enhances the overall energy efficiency of these industries.
- Use of Renewable Energy: When combined with renewable energy sources such as solar thermal or geothermal energy, HTEL becomes an emissions-free hydrogen production technology.
Challenges of High-Temperature Electrolysis
Despite its numerous advantages, HTEL faces several technical and economic challenges:
- High Material Requirements: The elevated temperatures demand materials with high thermal stability and corrosion resistance. Electrodes and ceramic membranes, which function as the electrolyte, must withstand extreme conditions for long-term operation.
- Complex System Design: Integrating thermal and electrochemical processes requires a complex system design. Efficient use of thermal energy and cooling of the electrolyzer cells pose technological challenges that complicate the development and implementation of HTEL systems.
- Scalability and Costs: Although HTEL offers great potential for large-scale hydrogen production, the production and operating costs remain relatively high due to the complex technology. Further technological advancements and cost reductions are essential for broader adoption.
Research and Development: Advances in High-Temperature Electrolysis
Research in the field of HTEL focuses on improving materials and optimizing system integration. The most promising research approaches include:
- Material Development: Developing new thermally stable and corrosion-resistant materials for electrodes and electrolytes is a key area of research.
- System Integration: Efficient use of heat sources, such as coupling HTEL with industrial waste heat or renewable energy sources, is another major focus. The goal is to reduce system complexity and maximize efficiency.
- Optimization of Operating Conditions: Through simulation and optimization of operating conditions such as temperature, pressure, and current density, HTEL efficiency can be further improved. This helps lower operating costs and makes the technology more economically attractive.