There is something big on the horizon – the European Health Data Space (EHDS)
“The European Health Data Space will help the EU to achieve a quantum leap forward in the way healthcare is provided to people across Europe. It will empower people to control and utilise their health data in their home country or in other Member States. It fosters a genuine single market for digital health services and products. And it offers a consistent, trustworthy and efficient framework to use health data for research, innovation, policy-making and regulatory activities, while ensuring full compliance with the EU's high data protection standards.” (EHDS Press Release, 3 May 2022)
All Member States will be required to participate. Analogue to the General Data Protection Regulation (GDPR) or Medical Device Regulation (MDR), this will be defined without a national legislation process but most likely delegating the detailed description on implementation strategies to the national levels. As this represents a true quantum leap for innovation in healthcare, this presents also challenges for data generating bodies such as hospitals, health insurance companies etc.
Our role in shaping health data spaces
Our team focuses on infrastructure-based topics to facilitate reproducible and high-qualitative secondary use of health data. Our related work over the past 20 years resulted in three categories of results directly related to activities in accordance with the EHDS.
1) Our extensive network of key stakeholders
We are in successful and continuous collaborations with all key stakeholders to shape future implementations of health data spaces, which includes academic institutions, public bodies, Austrian ministries, patient advocacy groups, cluster organisations and industry.
AIT is involved as key partner in leading national and international interest groups for data governance, data economy, interoperability etc., such as DIO, HL7, Gaia-X, EIT Health.
This enables us to get fist-hand updates on e.g., regulatory, and legal developments which serve as requirements for technical developments.
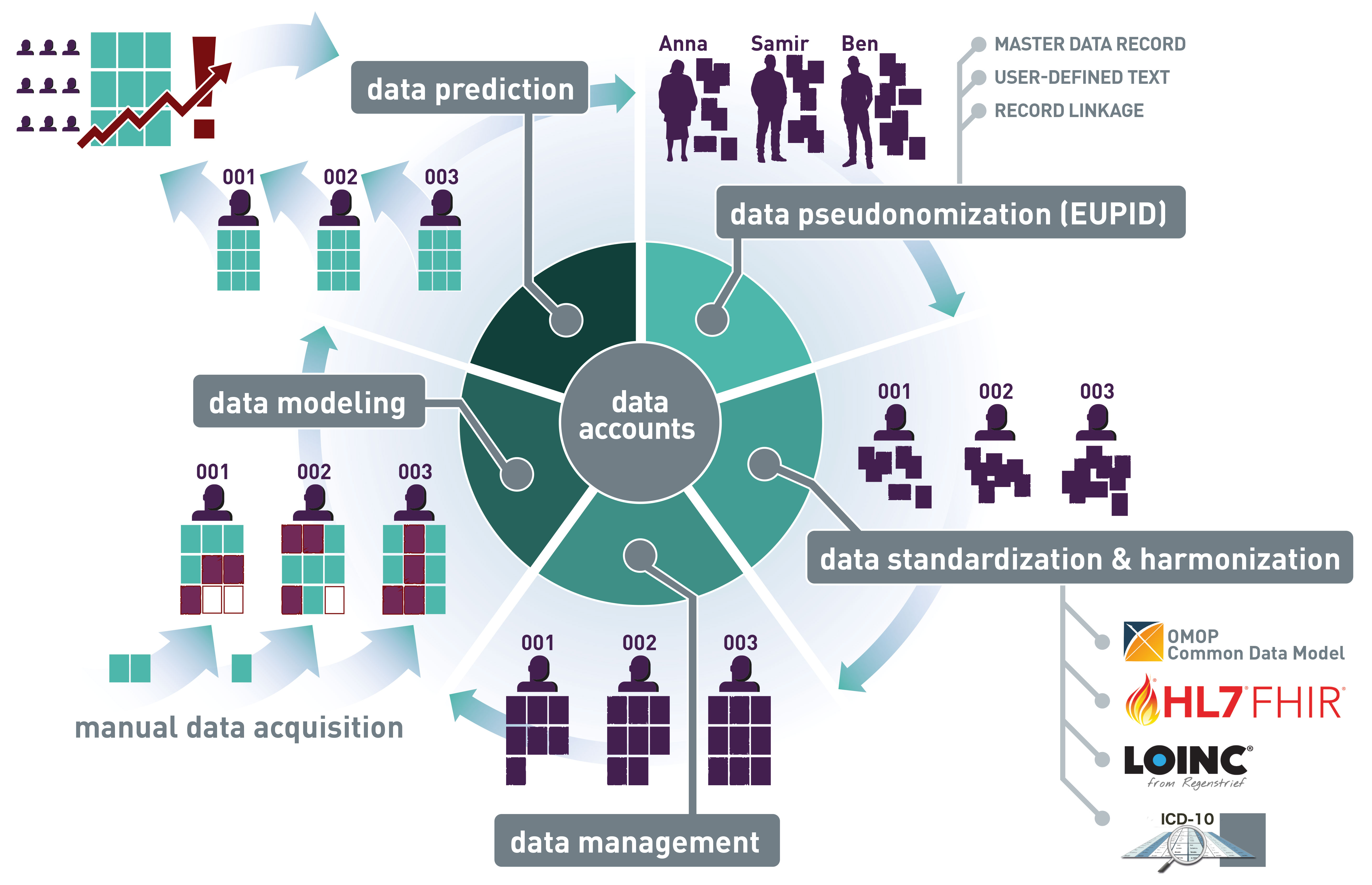
2) Our open technology framework
Wir bieten ein offenes Framework für eine föderierte, sichere Dateninfrastruktur, um innovative datengesteuerte Forschungs- und Entwicklungsaktivitäten zu ermöglichen. Durch die Verwendung unseres Frameworks für verteilte Datennodes stellen wir die erforderliche Werkzeugkette für die GDPR-konforme Vernetzung von Daten bereit, wobei wir Souveränität und Nachvollziehbarkeit sicherstellen. Unsere implementierten Datenschutztechnologien werden von zahlreichen Kunden umfassend getestet.
Our data nodes framework provides:
- Pseudonymization and record linkage for master data records and free-text records (e.g., clinical notes).
- Harmonization and Standardization. We build on a wide range of health standards including ISO11073, HL7 FHIR, the Clinical Data Architecture (CDA) and OMOP-CDM.
- Data curation and annotation. We offer convenient services for smart data quality checks and auditable data editing and classification.
- Collaborative data modelling. We offer collaborative work with networked data by providing an access-controlled model and feature store. Access can be managed by assigning different user-roles for different institutions.
- Model/algorithm as a Service. We provide the hosing of data apps based on self-developed models/algorithms or third-party applications.
Selected Projects
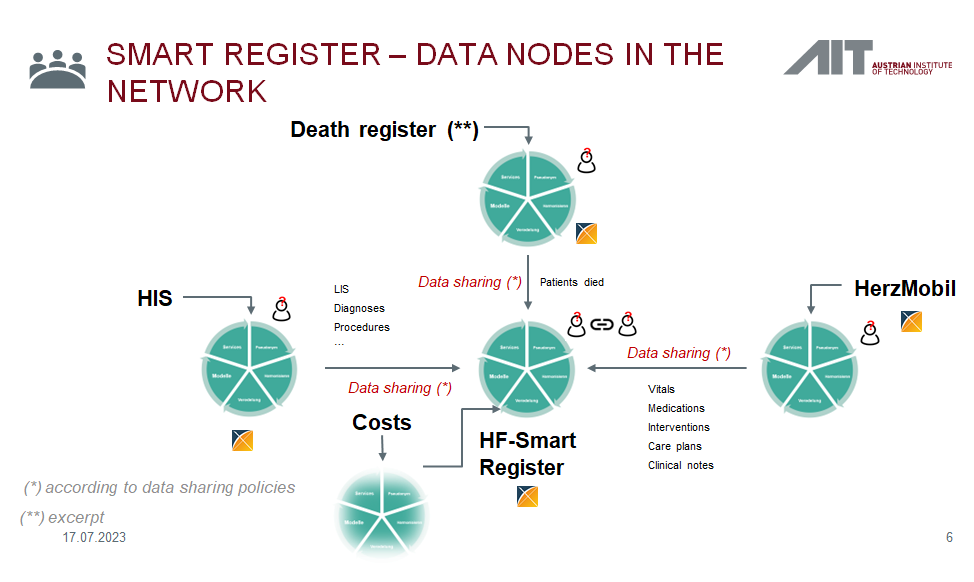
D4Health Tirol | We have integrated our federated secure data infrastructure to link data from hospitals, telemedicine services and Austria's death registry to support the application of machine learning and artificial intelligence in research and routine care | Funded by the state of Tyrol. Go-Live Dec 2022 |
PanCareSurPass | We are developing a Survivorship Passport for pediatric cancer survivors in Austria that links data from various sources in research and routine care in a privacy-friendly way. The solution will be linked to the Austrian electronic health record (EHR) ELGA. | H2020: "Implementation research for scaling up and transfer of innovative solutions involving digital tools for people-centred care" contract 11021892 |
European Joint Programme on Rare Diseases | As part of the EJP RD (European Joint Programme on Rare Diseases), we are researching methods for privacy-preserving record linkage (PPRL) based on our EUPID services, describing use cases for PPRL and researching methods for the interoperability of different PPRL solutions. | "European Commission - DG Research and Innovation" contact 11019651
|