Vienna - The H2020 European research project ELSAH (No 825549), led by the AIT Austrian Institute of Technology, has taken a decisive step in the development of wearable technologies. With the completion of its first-in-human clinical study, the project presents a ‘smart patch’ for painless continuous monitoring of health biomarkers.
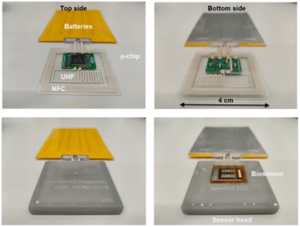
The innovative fully self-sustained system, known as the ELSAH-patch, combines a microneedle-based biosensor with electronics (microchip, battery and antennas) and enables painless, continuous monitoring of biomarkers such as glucose and lactate directly from the interstitial fluid of the skin. This technology promises to be an important step forward in preventive healthcare and management of physical training therapy especially for individuals with chronic diseases.
Two separate ELSAH-patch demonstrators were developed and successfully evaluated in the project:
- A technology demonstrator (codename Vienna), which integrated all the technology and scientific modules developed in the project into a functional non-clinical device evaluated in a laboratory environment. This demonstrator included printed electronics components. Successful in-vitro measurements of glucose and lactate were performed on the sensor interface and the measured data were transferred to the laptop or mobile phone using the integrated printed antennas. The upload of the measured data to the Lykon server infrastructure was successfully tested.
- A clinical study demonstrator (codename Graz) aligning with key medical device regulations and used for first-in-human trials. The developed microneedle-based biosensor and the microchip were integrated with commercial solutions for batteries (coin cells), antenna and interconnects (PCB technology). All components were fabricated, and quality controlled with full traceability through the distributed manufacturing flow across multiple European locations. The finished packaged devices were delivered to the project partner Medical University of Graz, Austria to perform the clinical study.
Dr. Giorgio C. Mutinati, Project Manager in the Molecular Diagnostics Competence Unit of the AIT Center for Health and Bioresources, emphasises the importance of this development: "Our goal was to create a technology that allows people to better understand and manage their health without the inconvenience and pain of traditional measurement methods. The results of our clinical study confirm that we have succeeded in this".
From Concept to Community: ELSAH's Clinical to User Pathway
The technology has been developed in collaboration with European partners, including research institutes, universities and companies from the SME and industrial sectors. The clinical evaluation of the patch involved 30 healthy volunteers and demonstrated the system's high level of accuracy and user-friendliness.
The innovation lies not only in the detection of the biomarkers, but also in the way the data is recorded and processed. "The secure wireless transmission of the measurement data to a smartphone app and then encrypted to a secure server infrastructure enables immediate analysis and interpretation of the health data. This opens up new possibilities for personalised health concepts and therapies," says Dr. Mutinati.
Another focus of the project is user acceptance. "The feedback from study participants has been consistently positive, particularly in terms of wearing comfort and ease of use. This is a crucial factor for the widespread acceptance and use of the technology in everyday life," adds Dr. Mutinati.
Charting the Path Forward: Advancing ELSAH into Daily Health Management
With the successful completion of the clinical study and the presentation of the integrated system, the vision is clear: ELSAH technology should play a central role not only in medical monitoring, but also in everyday life, helping people to manage their health independently and effectively.
The successful realisation of the ELSAH project and its promising future prospects symbolise significant progress in the development of wearable technologies. They also confirm Europe's position as an innovation leader in the global research sector. Given the wide range of possible applications of this technology, a new chapter in monitoring and proactive health care is now opening up, with the potential to sustainably improve everyday life and medical care at both individual and societal levels.
More Information: http://www.elsah.researchproject.at/
////////////////////////////////////////////////////////////////////////////////////////////////////////
Project Partners:
The Horizon 2020 research project (Grant No. 825549), supported by funding from the European Union, involved renowned partners:
- AIT Austrian Institute of Technology GmbH
Project coordination, development of the sensor surface modification using hydrogel-based biofunctional layers deposited with automated systems, system integration - DirectSens GmbH
Development of direct electron transfer (DET) enzymes, DET enzyme assay development - German Sport University Cologne
Development of use cases - Imperial College London
Validation of microneedle-based electrochemical dermal interstitial fluid biosensors - Infineon Technologies Austria AG
Development of an integrated microchip for biosensor control, data analysis and wireless communication - Centro Technológico LEITAT
Development of printed electronics (interconnects & antennas), Life cycle assessment. - LykonDX GmbH
Exploitation of the ELSAH-patch for healthcare and well-being - Sanmina Ireland Unlimited Company
ELSAH-patch system integration, patch applicator development, regulatory guidance - Saralon GmbH
Development of printed battery - Tyndall National Institute
Development of microneedles for dermal interstitial fluid biosensing based on flexible material technology - Medical University of Graz
First-in-human clinical study