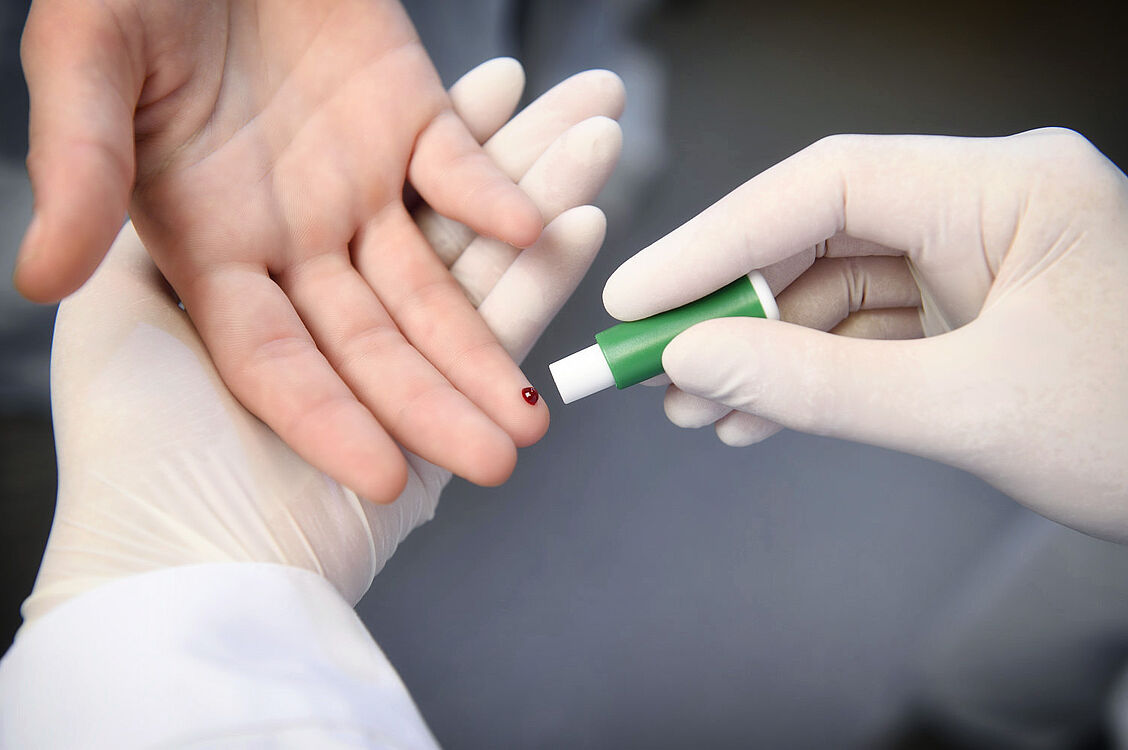
An antibody test for the SARS-CoV-2 virus that can be used in all medical laboratories is currently being developed by an Austrian consortium of three Viennese universities - the University of Natural Resources and Applied Life Sciences, the University of Veterinary Medicine and the Medical University of Vienna - and two application-oriented partners, including the AIT Austrian Institute of Technology. This test should be ready for use before the summer. In addition, a special test was developed at the AIT that even allows the determination of the antibody titer.